Abstract
Background: Gilteritinib, a highly selective fms-like tyrosine kinase 3 (FLT3)/AXL inhibitor, demonstrated strong antileukemic activity at doses ≥80 mg/day in patients with R/R AML with FLT3 mutations (FLT3mut+) enrolled in the CHRYSALIS phase 1/2 study (NCT02014558). We analyzed the impact of minimal residual disease (MRD) and achievement of complete remission/complete remission with partial hematologic recovery (CR/CRh) on overall survival (OS) in patients with FLT3mut+ R/R AML from the CHRYSALIS study.
Methods: Minimal residual disease was assessed by next-generation sequencing (NGS) using an Illumina® sequencing platform that quantified FLT3-ITD and total FLT3 alleles in a subgroup of FLT3mut+ patients with internal tandem duplication (ITD) mutations across all gilteritinib doses who had bone marrow samples available at baseline and at ≥1 post-baseline time point. The ITD variant allele frequency (VAF) was the FLT3-ITD to total FLT3 ratio. An ITD VAF ≤10−4 defined MRD-negative status. For FLT3 VAF, a capture-based NGS assay that included all exons of FLT3 was used. Sample DNA was used to generate whole genome libraries which were hybridized with a custom probe to capture target fragments that were sequenced on an Illumina® MiSeq platform. Treatment response was evaluated according to the CR/CRh rate. Complete remission was assessed according to the 2003 modified International Working Group criteria and CRh was defined as absolute neutrophil count >0.5 × 109/L and platelet count >50 × 109/L. The rate of CR/CRh has been used as an approval endpoint for R/R AML.
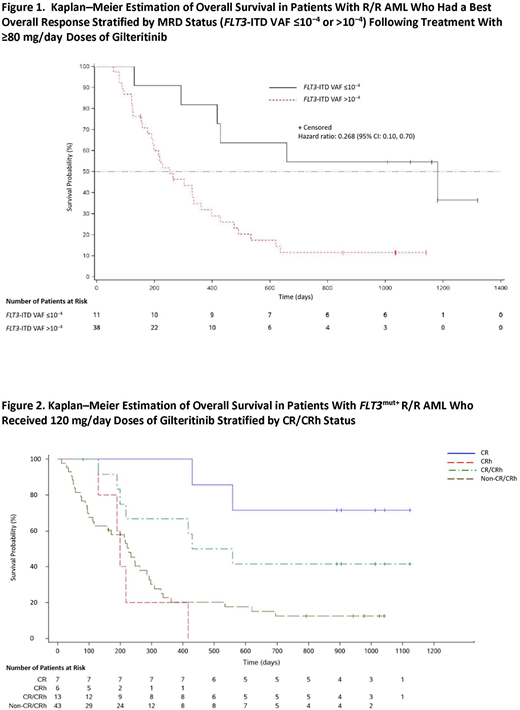
Results: The demographic and baseline characteristics of patients who were assessed for MRD and CR/CRh were representative of the entire CHRYSALIS R/R AML population. A total of 108 FLT3-ITDmut+ patients were analyzed for MRD; 95 of these patients had received ≥80 mg/day gilteritinib, which was shown to induce maximum FLT3 inhibition and antileukemic response. Eighty-two of the 95 patients were MRD-positive and 13 achieved MRD-negative status at any post-baseline time point. Of these 95 patients, 49 had a best overall response of composite complete remission (CRc; ie, CR plus CR with incomplete hematologic plus CR with incomplete platelet recovery) and 11 were MRD-negative. None of the patients who received <80 mg/day gilteritinib achieved MRD-negative status. Of the 46 patients who did not achieve CRc, two were MRD-negative. As seen in Figure 1, patients who had achieved CRc and were MRD-negative (n=11) had longer median OS (168.7 weeks; 95% CI: 41.7, not reached) than those who had achieved CRc and were MRD-positive (n=38; 36.1 weeks; 95% CI: 27.1, 51.7; P=.004). Excluding patients with an OS duration less than the median time to reach MRD-negative status, MRD-negative patients (n=12) had a median OS of 131.4 weeks (95% CI: 35.1, not reached) compared with MRD-positive patients (n=38) who had a median OS of 47.3 weeks (95% CI: 42.7, 61.1). Of the 95 patients who received ≥80 mg/day gilteritinib in the MRD analysis, 24 had a best overall response of CR/CRh. Of the 24 patients with CR/CRh, 10 (41.67%) were MRD-negative. Of the 71 patients without CR/CRh, three (4.2%) were MRD-negative.
Patients who received 120 mg/day gilteritinib were previously shown to have longer survival than patients in other dose cohorts. Of the 56 patients who received 120 mg/day gilteritinib, 13 achieved a best overall response of CR/CRh. As shown in Figure 2, patients who achieved CR/CRh had a median OS of 70.6 weeks (95% CI: 27.1, not reached) and a 52-week survival probability of 66.7% (95% CI: 33.7, 86.0) compared with patients who did not achieve CR/CRh who had a median OS of 32.4 weeks (95% CI: 16.0, 40.9) and a 52-week survival probability of 20.2% (95% CI: 9.5, 33.6).
Conclusions: Single-agent therapy with gilteritinib induced deep molecular responses, including MRD negativity, in heavily pretreatedpatients with FLT3-ITDmut+ R/R AML. Our results suggest a potential association between MRD-negative status and longer survival in patients withFLT3-ITDmut+ R/R AML. Additionally, patients who achieved CR/CRh appear to have both a higher rate of MRD negativity and longer OS than patients who did not achieve CR/CRh.
Perl:Daiichi Sankyo: Consultancy; Astellas: Consultancy; NewLink Genetics: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Actinium Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Arog: Consultancy; AbbVie: Membership on an entity's Board of Directors or advisory committees. Altman:Syros: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees, Other: payment to the institution to conduct clinical trial work; Janssen Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; GSK: Other: payment to the institution to conduct clinical trial work; FujiFilm: Other: payment to the institution to conduct clinical trial work; Pfizer: Other: payment to the institution to conduct clinical trial work; Cyclacel: Other: payment to the institution to conduct clinical trial work; Incyte: Other: payment to the institution to conduct clinical trial work; Genetech: Other: Payment to the institution to conduct clinical trial work; Ariad: Other: payment to the institution to conduct clinical trial work; Bayer: Other: payment to the institution to conduct clinical trial work; BMS: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Epizyme: Other: payment to the institution to conduct clinical trial work; Celator: Other: payment to the institution to conduct clinical trial work; Agios: Other: Payment to the institution to conduct the trial ; Astellas Pharma: Other; Immune Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Boeringer Ingelheim: Other: payment to the institution to conduct clinical trial work. Cortes:Novartis: Consultancy, Research Funding; Astellas Pharma: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Daiichi Sankyo: Consultancy, Research Funding; Arog: Research Funding. Smith:Astellas Pharma: Research Funding. Jurcic:Daiichi-Sankyo: Research Funding; Actinium Pharmaceuticals, Inc: Research Funding; Forma Therapeutics: Research Funding; Astellas: Research Funding; Genetech: Research Funding; Incyte: Consultancy; Syros Pharmaceuticals: Research Funding; Celgene: Research Funding; AbbVie: Consultancy, Research Funding; Kura Oncology: Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees. Ritchie:Incyte: Consultancy, Speakers Bureau; Bristol-Myers Squibb: Research Funding; NS Pharma: Research Funding; ARIAD Pharmaceuticals: Speakers Bureau; Astellas Pharma: Research Funding; Novartis: Consultancy, Other: Travel, Accommodations, Expenses, Research Funding, Speakers Bureau; Pfizer: Consultancy, Research Funding; Celgene: Consultancy, Other: Travel, Accommodations, Expenses, Speakers Bureau. Strickland:Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Boehringer Ingelheim: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Astellas Pharma: Consultancy; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees; Baxalta: Consultancy, Membership on an entity's Board of Directors or advisory committees; CTI Biopharma: Consultancy, Membership on an entity's Board of Directors or advisory committees; Tolero Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees; Sunesis Pharmaceuticals: Consultancy, Research Funding. Hill:Ligacept, LLC: Other: Shareholder. Rosales:Astellas Pharma: Employment. Bahceci:Astellas Pharma: Employment.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal